What Are Polyols and How Are They Used?
Polyoxyalkylene ether polyols, more commonly referred to as polyols, are organic compounds comprised of carbon bound to atoms of hydrogen and oxygen that form a hydroxyl compound. Poly- means many or multiple, and -ol refers to alcohol. Thus, they are alcohols made of multiple hydroxyl groups. Polyols are often defined by the number of hydroxyl groups: diols, which contain two hydroxyls; triols, which contain three; tetrols, which contain four, and so forth. They constitute a major raw material used in industrial manufacturing of plastics, foams, resins, and in foods, pharmaceutical, and medical products.
Polyols are classified according to their chemical composition. They are used as molecular chain extenders to increase molecular weight, forming a chemical backbone to build complex chains that produce a diverse range of industrial polymers. Polyols differ in viscosity, chemical composition, residual catalyst level and molecular weight. Viscosity ranges from free-flowing liquids to waxy solids. Polyols used in manufacturing and industrial applications include polymeric polyols, polyurethanes, polyesters, polycarbonate polyols and acrylic polyols.
Polyurethanes and other polymers are used in a wide range of industries because of their durability, ease of processing and performance. Products containing polyols include adhesives, flexible foams, rigid foam, foam insulation and paint. Industrial polyols are primarily made from nonrenewable resources, such as petroleum and petroleum derivatives. However, industry research is exploring ways to create polyols from renewable, bio-based materials, including castor oil, other vegetable oils and microalgae.
How Are Polyols Processed?
Crude polyols are produced using a chemical process that combines a starter, such as glycerin or sugar, with a catalyst to create an initiator. The initiator is combined with a reactant, such as propylene oxide, under controlled temperature and pressure conditions to create crude polyol. Crude polyols contain impurities that affect their economic value and ability to produce high-quality polymer products.
Impurities are often in the form of metal salts used as catalysts in the initial polyol production process. Concentrations of catalyst impurities usually range between 1,700 and 4,000 parts per million. Polyol filtration removes impurities, with the goal of reducing concentrations to approximately 5 ppm.
To purify polymers, crude polyols are mixed with water, adsorbents, such as magnesium silicate (MAGENSOL®), and solvents. This mixture is passed through a centrifugal discharge pressure leaf filter, which separates liquids and solids. There are two types of cake-discharge pressure leaf filtration systems: wet and dry. In dry-cake pressure leaf filters, the solids are dried after completion of the polymer filtration process. Spinning the filter leaves after drying dislodges the cakes, which drop to the bottom of the vessel into a receptacle within the filter. From there, cakes can be collected.
The handling of polyols and the disposal of spent adsorbent also presents challenges for the producer. After filtration, the resulting cake contains some significant amount of excess polyol combined with the adsorbent and filter-aid. These saturated cakes can be flammable or present other safety and ecological hazards. Polyols do not biodegrade so their waste should not be disposed of in landfills. To address this, the cakes are often washed with a solvent to remove excess polyols embedded in the solids and filters. Washing the cake after polyol filtration removes catalysts and other residual chemicals addressing economic, environmental and safety concerns, as well as increasing product recovery and therefore reducing production costs.
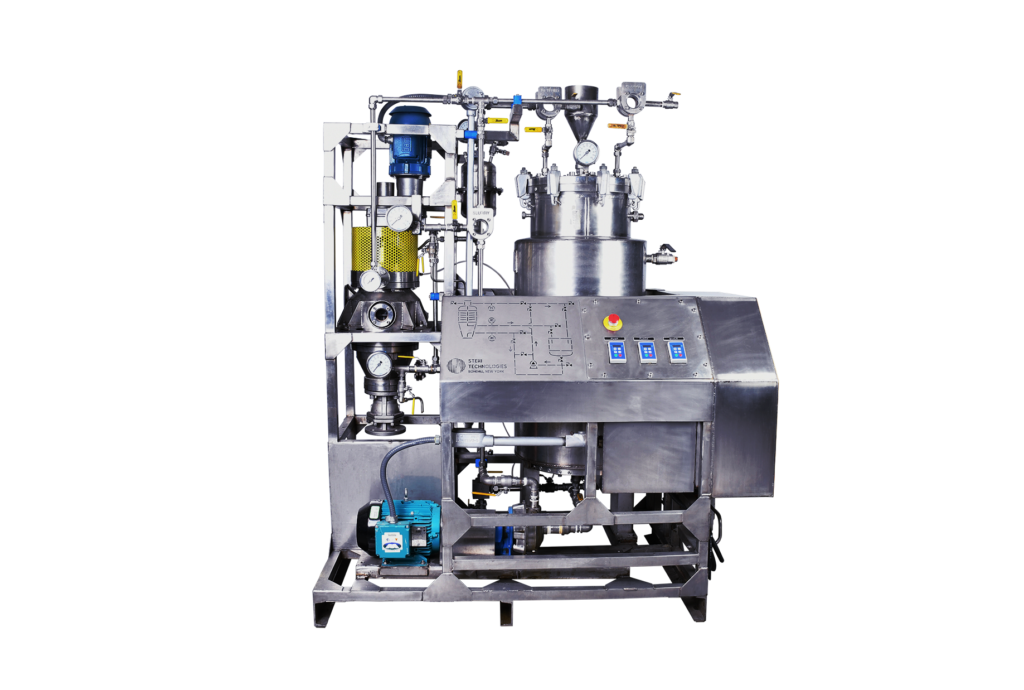
How Are Steri Funda Filters Used in the Polyol Production Process?
Steri’s Funda filter, also known as a pressure leaf filter, is a completely enclosed filtration unit built to ensure full containment of the contents. The pressure leaf filtration system is comprised of horizontally stacked filter plates fitted to a vertical shaft that bisects the vessel. Each filter leaf contains a plate, filtration screen and support screen secured by rings that hold the screens and entire assemblage in place. Spacing between rings and the plates is adjustable so that liquids can flow freely around and through the plates and the vessel.
The vessel is pressurized, which pushes the liquid through the plates, capturing the spent solid absorbent and impurities. The purified liquid, (i.e. filtrate) flows into a shaft and drains out through the bottom of the vessel. After the filtration cycle, unpurified liquids, called the heel, may remain at the bottom of the vessel. This liquid is pumped to the top of the filter and discharged to flow over the plates, ensuring maximum product recovery. This process is well-controlled so that the cakes on the plates are not disturbed.
After the final filtration, the cake remains on the filter plates and is washed with solvent or steam. The flow of liquid must be even so that it cascades down through all the plates. Cakes are dried using gas, typically nitrogen or even air in cases where the process allows. The liquid in the cakes is displaced using high pressure to force it through the capillaries to the surface of the cake. Both the filters and the cakes must be heated evenly during the drying cycle for complete removal of liquids. Any residual condensate must be drained from the filter before it is opened.
After the cakes are dried, the filter plate stack is rotated, which throws the dry cake to the vessel walls. The friable particles slide down the walls and drop to the bottom. After this process, the centrifugal discharge filter is depressurized and the cake discharge valve is opened, which allows the cake to exit the vessel. Cakes can be collected and disposed of properly.